|
HEART BLOCK |
Atrioventricular heart block results when impulses from the atrium (sinoatrial
node) fail to transmit to the ventricles. Normal electrical impulses originate
in the sinoatrial (SA) node, and travel to the atrioventricular (AV) node via
the purkinje system of fibers. Because the SA node is the intrinsic pacemaker
of the heart, in heart block it maintains a higher baseline rate resulting in
an atrial rate of 110-160 beats/minute and a ventricular rate from 40-80
beats/minute. It is therefore important to accurately compare atrial and
ventricular contraction rates to differentiate complete heart block from atrial
bigeminy.
CLASSIFICATION |
|
First degree block |
Prolonged PR interval resulting in a conduction delay. Not diagnosed in utero. |
|
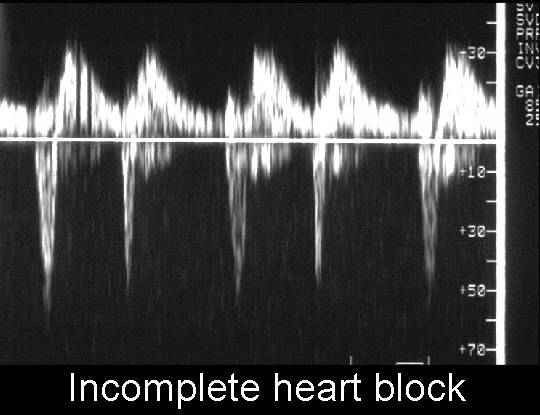
Second degree block |
Mobitz 1 - blockage of a single atrial beat |
|
Third degree block |
Complete heart block. Atrial and ventricular rates are
completely dissociated |
|
|
|
- ±50% of fetuses with complete heart block have complex structural abnormalities of the atrioventricular junction. Visceral heterotaxy with left atrial isomerism and atrioventricular discordance are the most common findings. Maldevelopment of the central portion of the heart where the AV node is located is the most common defect (1,2). Interruption of the electrical connection between the atria and ventricles result in independent function between the atria and ventricles. Situs anomalies that are part of atrial isomerism (asplenia or polysplenia) are often present.
- When atrioventricular valve regurgitation is present there is often associated non-immune hydrops. It is rare for a fetus with hydrops, complete heart block and structural heart disease to survive (1,2).
- Fetuses with complete heart
block and structurally normal hearts are usually affected by an
immunologically mediated process initiated by maternal autoantibodies
(3-5).
Sjögren syndrome (SLE) - anti-SSA, anti-SSB (anti-Ro and anti-La). These antibodies have a strong affinity for the fetal cardiac conduction system, provoking an intense immune response (6). They also bind to fetal myocytes and an immune myocarditis can be demonstrated (7). The antibodies cross the placenta and damage the His-Purkinje fibers of the conducting system, usually between 18-20 weeks gestation. - Fetal non-immune hydrops may result from a drop in fetal ventricular rate. Associated myocarditis may further reduce ventricular compliance.
REFERENCES |
- Machado MVL, Tynan MJ, Curry PVL et.al. Fetal complete heart block. Br Heart J 1988;60:512-515.
- Schmidt KG, Ulmer HE, Silverman NH et.al. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol 1991;17:1360-1366.
- Chamiedes L, Truex RC, Vetter V et.al. Association of maternal systemic lupus erythematosus with congenital complete heart block. N Engl J Med 1977;297:1204-1207.
- Scott JS, Maddison PJ, Taylor PV et.al. Connective tissue disease, antibodies to ribonucleoprotein and congenital heart block. N Engl J Med 1983;309:209-212.
- Taylor PV, Scott JS, Gerlis LM et.al. Maternal antibodies against fetal cardiac antigens in congenital complex heart block. N Engl J Med 1986;315:667-672.
- Lee LA, Coulter S, Erner S et.al. Cardiac immunoglobulin deposition in congenital heart block associated with maternal anti-Ro antibodies. Am J Med 1987;83:793-796.
- Horsfall AC, Venables PJW, Taylor PV et.al. Ro and LA antigens and maternal autoantibody idiotype in the surface of myocardial fibres in congenital heart block. J Autoimmun 1991;4:165-176.