THE
|
The normal coronary ostia |
|
|
|
|
Myocardial perfusion is maintained through the right and left coronary arteries (RCA and LCA, respectively) arising from the aortic root and the anterior descending branch of the left coronary artery (LAD). Superficial venous drainage enters the coronary sinus while a small portion drains directly into the cardiac chambers.
Embryologically the coronary vessels are formed through the union of intramyocardial blood islands and microvessels sprouting from the aortic root (1). The coronary arterial system is subject to great anatomic and functional variation and has a marked capacity for plasticity, which may be manifested in several ways (2). Myocardial oxygenation and metabolism are critically dependent on blood flow:
- Myocardial metabolism is almost exclusively aerobic and in the presence of adequate oxygen a variety of substrates, including carbohydrates, lactate and lipids, can be metabolized (3,4). In fetal life myocardial lactate oxidation constitutes the major cardiac energy source.
- High oxygen extraction (70%80%) and arteriovenous oxygen difference (14 mL/dL) in the resting state allows for little increase in oxygen delivery when cardiac demand increases. Such increased oxygen demand is almost exclusively met by augmentation of coronary blood flow (4,5).
Myocardial perfusion pressure is the primary driving force for coronary blood flow:
- It is determined by the pressure difference between the ascending aorta and the right atrium.
- Perfusion pressure is further modulated through vascular tone and extravascular resistance.
- Extravascular resistance is lowest during ventricular relaxation, therefore coronary blood flow is maximal in diastole (this unique feature distinguishes the coronary circulation from all other arterial vascular beds (6,7).
Autoregulation is a mechanism triggered by tissue hypoxia, which enhances oxygen delivery through augmentation of myocardial blood flow. This is achieved by modulation of precapillary sphincter tone at areas of greatest oxygen demand (6,8). Autoregulation is critical for the maintenance of optimal myocardial oxygenation to ensure normal cardiac performance (9,10). While autoregulation ensures short-term modulation of myocardial blood flow, long-term adaptation to chronic myocardial hypoxemia appears to involve vascular remodeling and structural alterations of the coronary microcirculation. This results in a significant increase in coronary blood flow reserve that can be recruited under circumstances of acutely worsening myocardial hypoxemia (5, 11, 12).
In fetal life, two parallel circulations exist:
- The umbilical vein/ductus venosus/left ventricle axis carries oxygenated blood to the myocardium and brain, while the vena cavae/right ventricle axis delivers blood to the placenta for re-oxygenation (13,14). Under resting conditions the myocardium is estimated to receive 8% of left ventricular output corresponding to approximately 3% of common cardiac output (13). These proportions may be even higher in the human fetus in which a larger proportion of umbilical venous blood reaches the left ventricle through the ductus venosus 914).
Through the parallel arrangement of the circulations coronary artery blood flow in the fetus may be augmented passively if there is redistribution of cardiac output towards the left ventricle. This may be observed in high placental resistance or ductus arteriosus constriction (15,16). There is also good evidence from animal and human data that coronary autoregulation is functional in fetal life (12-18). Acute hypoxemia and elevations in afterload are associated with a four- to fivefold increase in myocardial blood flow. This is analogous to the autoregulatory response seen in adult life. In addition the fetal coronary circulation also has the ability to adapt to chronic fetal conditions such as IUGR. The most striking changes in myocardial blood flow can be observed in fetuses subjected to acute hypoxemia after a period of chronic hypoxemia. In this setting of acute-on-chronic hypoxemia, maximal myocardial flow reserve is increased to 12-fold the basal flow, and is amongst the highest flows observed under any condition. This increase in flow reserve has been considered as evidence for coronary vascular remodeling and enhanced coronary reactivity to vasoactive agents (5,18).
|
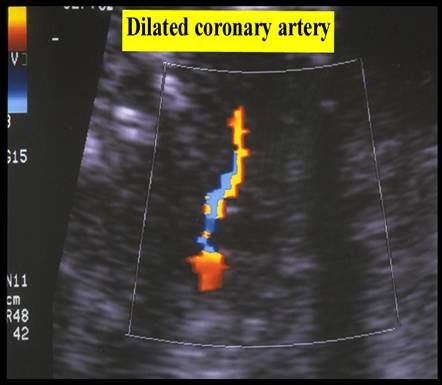
THE DILATED CORONARY
ARTERY |
Fetal coronary artery blood flow can be demonstrated in appropriately grown fetus from 32 weeks onward under favorable scanning conditions (fetal size and maternal body habitus) (17).
Coronary artery blood flow can be demonstrated at an earlier age in fetuses with IUGR and abnormal arterial and venous doppler findings. This is thought to be due to the “heart-sparing” effect, which results from acute impairment of oxygenation after a period of chronic compensation (18,19).
In a recent study (19), IUGR fetus that demonstrated coronary blood flow progressed to an advanced state of circulatory redistribution (20). Myocardial oxygen requirements are maintained primarily by regulation of coronary perfusion, which in turn is determined by the balance between coronary perfusion pressure and vascular resistance. Maximum myocardial flow reserve, is increased by chronic hypoxemia in the fetal lamb (15).
The use of coronary blood flow as a critical outcome predictor is limited.
|
|
|
FETAL ANEMIA |
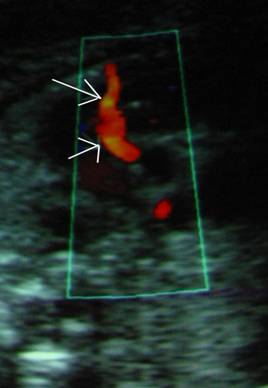
Visualization of coronary artery blood flow may be possible in acute fetal anemia in situations such as fetomaternal hemorrhage (21), but also in more chronic anemia with fetal hydrops. Coronary artery peak blood flow velocities are significantly elevated and correlate with the fetal hemoglobin level. With correction of fetal anemia, coronary blood flow is no longer discernable by either color flow mapping or spectral Doppler.
DUCTUS ARTERIOSUS CONSTRICTION |
Sudden ability to visualize coronary artery blood flow was also observed in three fetuses with severe ductus arteriosus constriction following indomethacin therapy for preterm labor. All three cases shared the same circulatory features. The ductus arteriosus pulsatility index was below 1.1, there was elevation of precordial venous indices and holosystolic tricuspid regurgitation. Visualization of coronary blood flow was no longer possible once these findings resolved with the discontinuation of indomethacin. In this setting it is likely that coronary blood flow augmentation was precipitated by increased myocardial oxygen demand in view of the increased afterloada situation that is analogous to experiments in the sheep fetus (17).
FETAL BRADYCARDIA |
Transient 'brain- and heart-sparing' phenomena were observed in a 30-week fetus following a 12-minute bradycardia after umbilical fetal blood sampling. Sudden visualization of coronary blood flow, 'brain sparing' and highly pulsatile precordial venous flow persisted for a long period after the bradycardia resolved (18). It is likely that this phenomenon is due to a combination of increased afterload, decreased cardiac output, elevated venous pressure and hypoxemia. With the resolution of heart and brain sparing venous Doppler findings improve suggesting no residual cardiac dysfunction.
CRITICAL AORTIC STENOSIS |
Development of fetal heart sparing has also been reported in severe left ventricular outflow tract obstruction with nonimmune hydrops due to critical aortic stenosis (22). In this case visualization of coronary blood flow became possible for the first time at 39 weeks and was also associated with functional closure of the foramen ovale. It is likely that the underlying mechanism represents the combined effects of increased cardiac work and a relative decrease of myocardial perfusion, thus exacerbating myocardial hypoxemia.
IDIOPATHIC ARTERIAL CALCIFICATION |
The idiopathic arterial calcification has an unknown etiology and is characterized by generalized arterial calcification and stenoses especially of the walls in the arterial trunk of the pulmonary artery and aorta (23,24). Most commonly the coronary arteries are also affected, but peripheral arteries of gastrointestinal tract, liver, kidneys, brain, extremities, and placenta may also be involved. Severe myocardial dysfunction may cause fetal hydrops, tissue ischemia and fetal death in the late second or third trimesters (24,25). In less severe cases, especially in the absence of hydrops, palliative treatment postpartum includes steroids and bisphosphonates in order to arrest or delay the disease progression(24,25). However, most infants with idiopathic arterial calcification die within the first year of life due to complicating cardiopulmonary failure, severe hypertension, renal infarction, peripheral gangrene, and bowel infarction (24).
REFERENCES |
1. Tomanek RJ. Formation of the coronary vasculature: a brief review. Cardiovasc Res 1996; 31: E46-E51
2. Coles JG, Freedom RM, Lightfoot NE, Dasmahapatra HK, Williams WG, Trusler GA, Burrows PE. Long-term results in neonates with pulmonary atresia and intact ventricular septum. Ann Thorac Surg 1989; 47: 213-7
3. Engelmann GL, Dionne CA, Jaye MC. Acidic fibroblast growth factor and heart development. Role in myocyte proliferation and capillary angiogenesis. Circ Res 1993; 72: 7-19
4. Spahr R, Probst I, Piper HM. Substrate utilization of adult cardiac myocytes. Basic Res Cardiol 1985; 80 (Suppl. 1): 53-6
5. Reller MD, Morton MJ, Giraud GD. Maximal myocardial flow is enhanced by chronic hypoxaemia in late gestational fetal sheep. Am J Physiol 1992; 263: H1327-1329
6. Thornburg KL, Reller MD. Coronary flow regulation in the fetal sheep. Am J Physiol 1999; 277: R1249-1260
7. Ofili EO, Labovitz AJ, Kern MJ. Coronary flow velocity dynamics in normal and diseased arteries. Am J Cardiol 1993; 14: 71-78
8. Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res 1996; 32: 668-678
9. Barnea O, Santamore WP. Coronary autoregulation and optimal myocardial oxygen utilization. Basic Res Cardiol 1992; 87: 290-301
10. Hoffman JIE. Maximal coronary blood flow and the concept of coronary vascular reserve. Circulation 1984; 70: 153-159
11. Campbell
SE, Kuo CJ, Hebert B, Rakusan
K,
12. Holmes G, Epstein ML. Effect of growth and maturation in a hypoxic environment on maximum coronary flow rates of isolated rabbit hearts. Pediatr Res 1993; 33: 527-532
13. Rudolph AM. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res 1985; 57: 811-821
14. Kiserud T, Rasmussen S, Skulstad S. Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am J Obstet Gynecol 2000; 182: 147-153
15. Reller MD, Morton MJ, Giraud GD, Wu DE, Thornburg KL. Severe right ventricular pressure loading in fetal sheep augments global myocardial blood flow to submaximal levels. Circulation 1992; 86: 581-588
16. Gembruch U, Baschat AA. Circulatory effects of acute bradycardia in the human fetus as studied by Doppler ultrasound. Ultrasound Obstet Gynecol 2000; 15: 424-427
17. Baschat AA, Gembruch U, Reiss I, Gortner L, Diedrich K. Demonstration of fetal coronary blood flow by Doppler ultrasound in relation to arterial and venous flow velocity waveforms and perinatal outcomethe 'heart-sparing effect'. Ultrasound Obstet Gynecol 1997; 9: 162-172
18. Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Coronary blood flow visualization indicates cardiovascular compromise in IUGR fetuses. Ultrasound Obstet Gynecol 2000; 16: 425-431
19. Baschat AA, Gembruch U, Harman CR. Coronary blood flow in fetuses with intrauterine growth restriction. J Perinat Med 1998;26:143-146.
20. Rizzo G, Capponi A, Pietropolli A et.al. Fetal cardiac and extracardiac flows preceding intrauterine demise. Ultrasound Obstet Gynecol 1994;4:139-142.
21. Baschat AA, Harman CR, Alger LS, Weiner CP. Fetal coronary and cerebral blood flow in acute fetomaternal hemorrhage. Ultrasound Obstet Gynecol 1998; 12: 128-131
22. Schmider A, Henrich W, Dahnert I, Dudenhausen JW. Prenatal therapy of non-immunologic hydrops fetalis caused by severe aortic stenosis. Ultrasound Obstet Gynecol 2000; 16: 275-278
23. Hajdu J, Marton T, Papp C, Hruby E, Papp Z. Calcification of the fetal heartfour case reports and a literature review. Prenat Diagn 1998; 18: 1186-1190
24.
25. Juul S, Ledbetter D,